A vaccine for Ebola which has completed successful trials in Guinea and Sierra Leone means the virus should never again be able to wreak the havoc it did during the recent epidemic in west Africa, say scientists.
More than 11,000 people died in the outbreak, which began unnoticed in December 2013 and spread across the region, infecting at least 28,600 people and triggering a global response, including a race to get an effective vaccine tested and into use.
Final results for the vaccine that was rushed into trials in Guinea and later Sierra Leone show that it was highly effective against one of the most lethal known pathogens in existence. Ten days after vaccination, none of the trial subjects developed Ebola virus disease. The very few who did, in the days immediately following vaccination, are thought to have been infected already.
“While these compelling results come too late for those who lost their lives during west Africa’s Ebola epidemic, they show that when the next Ebola outbreak hits, we will not be defenceless,” said Dr Marie-Paule Kieny, the World Health Organisation’s assistant director general for health systems and innovation, and the study’s lead author.

Merck, Sharp & Dohme, the company manufacturing the vaccine, has received permission to go through fast-track procedures for a licence from the US and European regulatory authorities. It has committed to making 300,000 doses that will be ready for any emergency even before formal approval, with $ 5m (£4m) in funding from Gavi, the Vaccine Alliance.
The trial began in the coastal region of Basse-Guinée, which still had cases in 2015, even though the numbers were abating across the region. Writing in the Lancet medical journal, the scientists say it was not easy.
“A devastating outbreak of Ebola virus disease is clearly not the ideal situation for doing a vaccine trial. The healthcare system in Guinea was strained, potential trial participants were worried about a candidate vaccine made by foreign people, and the Ebola virus disease response teams were facing security issues,” they write.
They collaborated closely with the government and local authorities in Guinea and chose a “ring vaccination” design for the trial, which was unusual but had been successful in helping stamp out smallpox decades ago.
When a new case of Ebola was diagnosed, the teams offered vaccination to everybody who had been in contact with that person in the previous three weeks, from family to friends and neighbours. They also offered vaccination to the closest contacts of those contacts. This cluster – or ring – amounted to around 80 people on average. Altogether, 117 such rings or clusters were identified. At first, adults were randomly assigned to get the vaccine immediately or three weeks later, but when it became clear that the vaccine was protecting most people, everybody was offered immediate vaccination, including children.
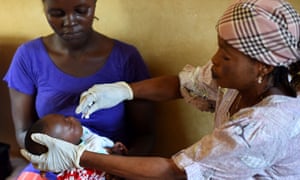
Among the 5,837 people who received the vaccine, still known only as rVSV-ZEBOV, no Ebola cases were recorded 10 days or more after vaccination. Among those who were not vaccinated, there were 23 cases. There were very few serious side-effects – one case of fever and one of anaphylaxis (allergic reaction) thought to be related to the vaccine.
The authors of the study say the ring design was also helpful in ending the outbreak and suggest it could be a useful way to tackle the disease in future.
Co-author John Edmunds, professor of infectious disease modelling at the London School of Hygiene and Tropical Medicine, whose team helped design the trial, said: “This novel and historic trial, conducted under the most difficult of circumstances, has demonstrated that the rVSV-ZEBOV vaccine is safe and effective. When Ebola strikes again we will be in a much better position to offer help to affected communities, as well as protect the brave volunteers who help control this terrible disease.”
Jeremy Farrar, director of the Wellcome Trust, which supported the trial, said the outcome was “simply remarkable” and demonstrated what was possible even in the midst of a raging epidemic.“We’ve shown that by working collaboratively, across international borders and sectors, we can develop and test vaccines rapidly and use them to help bring epidemics to an end,” he said.
“Had a vaccine been available earlier in the Ebola epidemic, thousands of lives might have been saved. We have to get ahead of the curve and make promising diagnostics, drugs and vaccines for diseases we know could be a threat in the future. My hope is that this success story provides the inspiration we need to make this happen and change the way the world prepares for epidemics.”
Dr Sakoba Kéita, coordinator of the Ebola response and director of the National Agency for Health Security in Guinea, said: “Ebola left a devastating legacy in our country. We are proud that we have been able to contribute to developing a vaccine that will prevent other nations from enduring what we endured.”
Ebola vaccine is safe and effective, scientists declare after trials
Hiç yorum yok:
Yorum Gönder